Coagulation and flocculation are an essential part of drinking water treatment as well as wastewater treatment. This article provides an overview of the processes and looks at the latest thinking. Material for this article was largely taken from reference1.
Coagulation and flocculation are essential processes in various disciplines. In potable water treatment, clarification of water using coagulating agents has been practiced from ancient times. As early as 2000 BC the Egyptians used almonds smeared around vessels to clarify river water. The use of alum as a coagulant by the Romans was mentioned in around 77 AD. By 1757, alum was being used for coagulation in municipal water treatment in England.
In modern water treatment, coagulation and flocculation are still essential components of the overall suite of treatment processes – understandably, because since 1989 the regulatory limit in the US for treated water turbidity has progressively reduced from 1.0 NTU in 1989 to 0.3 NTU today. Many water utilities are committed to consistently producing treated water turbidities of less than 0.1 NTU to guard against pathogen contamination.
Coagulation is also important in several wastewater treatment operations. A common example is chemical phosphorus removal and another, in overloaded wastewatertreatment plants, is the practice of chemically enhancing primary treatment to reduce suspended solids and organic loads from primary clarifiers.
The Coagulants
The commonly used metal coagulants fall into two general categories: those based on aluminum and those based on iron. The aluminum coagulants include aluminum sulfate, aluminum chloride and sodium aluminate. The iron coagulants include ferric sulfate, ferrous sulfate, ferric chloride and ferric chloride sulfate. Other chemicals used as coagulants include hydrated lime and magnesium carbonate.
The effectiveness of aluminum and iron coagulants arises principally from their ability to form multi-charged polynuclear complexes with enhanced adsorptioncharacteristics. The nature of the complexes formed may be controlled by the pH of the system.
When metal coagulants are added to water the metal ions (Al and Fe) hydrolyze rapidly but in a somewhat uncontrolled manner, forming a series of metal hydrolysis species. The efficiency of rapid mixing, the pH, and the coagulant dosage determine which hydrolysis species is effective for treatment.
There has been considerable development of pre-hydrolyzed inorganic coagulants, based on both aluminum and iron to produce the correct hydrolysis species regardless of the process conditions during treatment. These include aluminum chlorohydrate, polyaluminum chloride, polyaluminum sulfate chloride, polyaluminum silicate chloride and forms of polyaluminum chloride with organic polymers. Iron forms include polyferric sulfate and ferric salts with polymers. There are also polymerized aluminum-iron blends.
The principal advantages of pre-polymerized inorganic coagulants are that they are able to function efficiently over wide ranges of pH and raw water temperatures. They are less sensitive to low water temperatures; lower dosages are required to achieve water treatment goals; less chemical residuals are produced; and lower chloride or sulfate residuals are produced, resulting in lower final water TDS. They also produce lower metal residuals.
Pre-polymerized inorganic coagulants are prepared with varying basicity ratios, base concentrations, base addition rates, initial metal concentrations, ageing time, and ageing temperature. Because of the highly specific nature of these products, the best formulation for a particular water is case specific, and needs to be determined by jar testing. For example, in some applications alum may outperform some of the polyaluminum chloride formulations1.
PoIymers are a large range of natural or synthetic, water soluble, macromolecular compounds that have the ability to destabilize or enhance flocculation of the constituents of a body of water.
Natural polymers have long been used as flocculants. For example, Sanskrit literature from around 2000 BC mentions the use of crushed nuts from the Nirmali tree (Strychnos potatorum) for clarifying water – a practice still alive today in parts of Tamil Nadu, where the plant is known as Therran and cultivated also for its medicinal properties. In general, the advantages of natural polymers are that they are virtually free of toxins, biodegradable in the environment and the raw products are often locally available. However, the use of synthetic polymers is more widespread. They are, in general, more effective as flocculants because of the level of control made possible during manufacture.
Important mechanisms relating to polymers during treatment include electrostatic and bridging effects. Figure 1 shows schematic stages in the bridging mechanism. Polymers are available in various forms including solutions, powders or beads, oil or water-based emulsions, and the Mannich types. The polymer charge density influences the configuration in solution: for a given molecular weight, increasing charge density stretches the polymer chains through increasing electrostatic repulsion between charged units, thereby increasing the viscosity of the polymer solution.

Figure 1. Stages in the bridging mechanism: Dispersion; (ii) Adsorption; (iii) Compression or settling down (see inset); (iv) Collision2
One concern with synthetic polymers relates to potential toxicity issues, generally arising from residual unreacted monomers. However, the proportion of unreacted monomers can be controlled during manufacture, and the quantities present in treated waters are generally low.
Removal of Natural Organic Matter
Natural organic material (NOM) is usually associated with humic substances arising from the aqueous extraction of living woody substances, the solution of degradation products in decaying wood and the solution of soil organic matter. These substances are objectionable for a number of reasons: they tend to impart color to waters; they act as a vehicle for transporting toxic substances and micro-pollutants, including heavy metals and organic pollutants; and they react with chlorine to form potentially carcinogenic by-products.
The degree to which coagulation can remove organic material depends on the type of material present, as shown in Figure 2. The specific ultraviolet absorption (SUVA) is related to the concentration and type of dissolved organic carbon (DOC) present, as follows:
SUVA = UV254/DOC (l/mg m)
Where: UV254 is the ultraviolet absorbance measure at a wavelength of 253.7 nm, after filtration through 0.45-µm filters (m-1); DOC is the dissolved organic carbon measured after filtration through 0.45-µm filters (mg/l).
In general, lower molecular weight species such as the fulvic acids are more difficult to remove by coagulation. Higher molecular weight humic acids tend to be easier to remove.
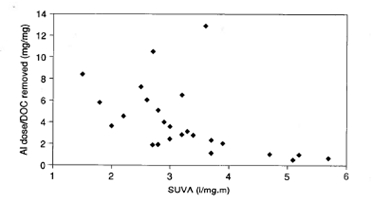
Figure 2. Influence of SUVA on alum (as Al) requirements for DOC removal
The United States Environmental Protection Agency (US EPA) introduced enhanced coagulation for the removal of NOM. Enhanced coagulation is an elaboration of long-practiced techniques for removing organic color by coagulation. It requires the removal of NOM material, while still achieving good turbidity removal. These dual objectives can be met by selecting the best coagulant type, applying the best coagulant dosage and adjusting the pH to a value where best (or adequate) overall coagulation conditions are achieved.
The enhanced coagulation approach recognizes that the constituents of any given water govern the practical degree of treatment achievable. Therefore, a water-specific point of diminishing returns (PODR) is identified, at which a coagulant increment (10 mg/l for alum) results in a TOC removal increment of less than 0.3 mg/l.
Organics removal and enhanced coagulation are effective with traditional coagulants like aluminum sulfate, ferric chloride and ferric sulfate, as well as formulations like polyaluminum chloride (PACl) and acid alum. Acid alum formulations are aluminum sulfate with 1 to 15-percent free sulfuric acid. Their effectiveness with TOC removal applications is due to the enhanced depression of pH.
TOC or NOM reductions depend on the type and dosage of coagulant, the pH, temperature, raw water quality and NOM characteristics. In general, the optimum pH for ferric salts is in the range 3.7 to 4.2, and for aluminum sulfate in the range 5.0 to 5.5.
In some cases, the removal of lower weight organics has been improved by supplementing treatment with metal coagulants with powdered activated carbon (PAC). In one case with raw water TOC of 2.4 mg/l, a combination of an alum-polymer blend coagulant at 25 mg/l with PAC at 10 mg/l was optimal to achieve a 39-percent TOC reduction.
In another case, a water with a low humic content and low SUVA (1.43 l/mg.m) was treated with 65 mg/l FeCl3 and 23 mg/l PAC. Fifty-six percent of the TOC was non-humic and 46-percent of the TOC had molecular weights less than 1,000.
Pathogen Removal
The U.S. EPA surface water treatment rule requires 99.9-percent (3-log) Giardia removal or inactivation, and at least 99-percent (2-log) removal of Cryptosporidium. Adequately designed and operated water treatment plants, with coagulation, flocculation, sedimentation and filtration are assigned a 2.5-log removal credit for Giardia, leaving only 0.5-log inactivation to be achieved by disinfection.
Coagulation and flocculation, with dissolved air flotation (DAF) for clarification, has achieved average log removals of Giardia and Cryptosporidium of 2.4 and 2.1, respectively. Optimum coagulation conditions were governed by turbidity and NOM removal requirements, rather than by pathogen removals. Overall Giardia andCryptosporidium removals, including the filtration step were approximately 5-log.
Cryptosporidium oocyst surfaces are believed to consist of polysaccharide layers. The negative charge carried by the oocysts is believed to arise from carboxylic acid groups in surface proteins. Removal of Cryptosporidium using alum coagulation appears to be by a sweep floc mechanism. Zeta potential measurements suggest that removal does not appear to be by a charge neutralization mechanism at lower DOC concentrations. At higher DOC, it appears that the mechanism is mediated by a NOM-assisted bridging between aluminum hydroxide and oocyst particles.
Significant virus removals have been reported using metal coagulants and polyelectrolytes. Removals of up to 99.9% have been reported for both aluminum and ferric salts. Various polyelectrolytes (cationic) have effected removals of greater than 99% but have the disadvantage that if other material is present in the form of color, turbidity, and COD, removal of such material is poor. Using metal coagulants and poyelectrolytes conjointly has the advantage that better floc characteristics are produced. If a variety of substances are present in water, it is possible that the use of both metal coagulants and polyelectrolytes will effect a higher overall removal. However, this very much depends on the conditions pertaining for each case. When using polyelectrolytes as flocculant aids, floc formation improves but does not appear to improve virus removals beyond those achieved using metal coagulants alone.
Viruses are essentially DNA (deoxyribonucleic acid) or RNA (ribonucleic acid) units contained within a protein coat. The destabilization mechanism involves coordination reactions between metal coagulant species and carboxyl groups of the virus coat protein. Because of the similarity of the destabilization mechanisms for organic color and viruses, optimum removals tend to occur at similar pH values. The optimum pH for virus removal with aluminum sulfate has been found to be in the region of 5.0 with percentage virus removals in the range 97.7 to 99.8%. Using a cationic polyelectrolyte as flocculant aid, virus and turbidity removals were increased to 99.9 and 98.5% respectively.
Metal coagulants or polyelectrolytes do not fully inactivate viruses. Therefore, a potential health hazard exists with the ultimate disposal of water treatment plantsludges. Furthermore, complete virus removal by destabilization with metal coagulants has not been reported. For a safe drinking water, disinfection of the water before distribution is required.
However, there is some inactivation that accompanies virus removal by coagulation. Some reports have shown that the infectious virus concentration only recovers partially after re-dissolution of aluminum hydroxide precipitates. This phenomenon has been interpreted as virucidal activity of the aluminum. PACl coagulants appeared to have a higher virucidal activity compared with alum. The presence of NOM in waters appears to inhibit the virucidal activity of the aluminum.
Removal of Inorganics
Coagulation operations can be useful in some cases for the removal of inorganics. Examples of successful applications are copper and mercury reductions from wastewaterplant effluents. Two applications discussed in more detail below are arsenic and fluoride removals in potable water treatment:
Arsenic removal
Arsenic is a commonly occurring toxic element and long term exposure to arsenic is injurious to health. The World Health Organization in 1993 reduced the arsenic limit for drinking water from 50 mg/l to 10 mg/l. In the United States, since passage of the Safe Drinking Water act in 1976, the maximum allowable arsenic concentration in drinking water was 50 mg/l. In 2002 this limit was lowered to 10 mg/l. Some states may adopt arsenic limits below the Federal limit. For example, the State of New Jersey in 2005 announced a plan to adopt a 5 mg/l limit and the State of California appeared to be considering an arsenic limit of approximately 4 mg/l.
Arsenic is stable in several oxidation states, under different redox conditions in water. However, when present in groundwater, arsenic occurs mostly in the forms of arsenite, As(III) and arsenate, As(V). As(III) is usually the predominant form in many groundwaters since it is more likely to be found in oxygen free (eerobic) conditions. As(V) is more common in aerobic waters. In general, As(V) is more readily removed than As(III).
There are various technologies used for removing arsenic from drinking water. These include adsorption on granular iron based media; adsorption on ion exchange resins;adsorption on activated alumina; coprecipitation in iron removal plants; coagulation with alum or ferric followed by conventional filtration; and coagulation with ferric followed by membrane filtration. Some studies have shown that the removal of arsenic by coagulation is more economical than other treatment alternatives.
With coagulation for arsenic removal, iron based coagulants are generally more effective than aluminum coagulants. Iron coagulants added to water hydrolyze to form ferric hydroxide with a net positive charge. This net positive charge is a function of pH. As the pH decreases, the number of positively charged sites on the ferric hydroxide particles increases. Arsenate, As(V) is an anion and since it is negatively charged, it will adsorb to the positively charged ferric hydroxide particles by surface complexation. Arsenic removal is generally optimized at pH values of less than approximately 7.).
Of the aluminum coagulants, the efficiencies of arsenic(V) removals are generally in the order polyaluminum chloride > polyaluminum sulfate > aluminum chloride > aluminum sulfate. Best results are obtained obtained at a pH of 5.5. Arsenic removals have ranged from approximately 59 to 99-percent at dosages of 0.8 to 1.9 mg/l as Al, with sedimentation followed by filtration.
Using ferric coagulants efficiencies of arsenic(V) removals are in the order polyferric chloride > polyferric sulfate > feric chloride > ferric sulfate. Best results are obtained at a pH of 5.5. As(V) removals ranged from approximately 70 to 99.6-percent at dosages of 1.7 to 3.8 mg/l as Fe, with sedimentation followed by filtration.
Figure 3 presents a synthesis of arsenic removal results obtained by a number of workers.
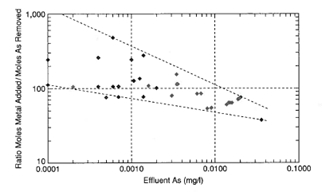
Figure 3. Synthesis of arsenic removal results
Fluoride removal
In 1975, the EPA named fluoride as a contaminant in the National Interim Primary Drinking Water Regulations. A Maximum Contaminant Level (MCL) was set at 1.4 – 2.4 mg/l to prevent dental fluorosis and more serious effects. To balance the benefits of fluoride for dental health, the deleterious effects of ingesting too much fluoride, and the costs of removing high concentrations of naturally occurring fluoride, the EPA in 1985 issued a new MCL of 4 mg/l for fluoride, with a secondary MCL of 2 mg/l. Systems with fluoride levels between 2 mg/l and 4 mg/l must provide the public with information about possible tooth discoloration.
The best available technologies for fluoride removal from water are generally considered to be activated alumina adsorption and reverse osmosis. However, in some cases, fluoride removal by aluminum coagulation has been shown to be cost effective. It appears that several aluminum based coagulants are equally effective, based on the aluminum content added for treatment. Fluoride removal using aluminum based coagulants is strongly affected by pH and aluminum dosage. Optimum pH varies from 6.0 to 7.5. A further factor is the residual aluminum remaining after treatment. Higher aluminum dosages often produce lower residual aluminum due to adsorption of fluoride to aluminum hydroxide flocs, rather than producing aluminum-fluoride complexes that remain in solution.
Aluminum dosages are generally high for appreciable fluoride removals. For example, to reduce fluoride from 3.6 mg/l to 1.8 mg/l, the aluminum dosage was 18 mg/l as Al, or 10 mg Al per mg fluoride removed, at an optimum pH of 6.5. To further reduce the fluoride to 1.0 mg/l, a dosage of 12 mg Al per mg fluoride removed was required.
Chemical Phosphorus Removal
In many sensitive catchment areas, chemical phosphorus removal is also required for wastewater treatment. Figure 4 shows the general relationship between effluent residual phosphorus concentration and the ratio of metal added to P removed. Inordinate dosages, beyond stoichiometric, are required to achieve very low effluent concentrations.
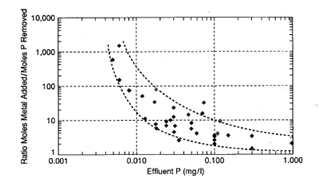
Figure 4. Synthesis of phosphorus removal results
Within the stoichiometric range of phosphorus removal, there is a tightening of the optimal pH range as the metal coagulant dosage increases. However, beyond the stoichiometric range, when final phosphorus concentrations are progressively lower, the pH range widens again, towards the side of higher pH. For example, with alum the optimum pH range for effluent P concentrations down to approximately 0.2 is 5.5 to 6.0. However, as the Al [emoticon_tongue] ratio is increased for lower P concentrations, the required pH range widens to 6.0 to 7.0.
Within the stoichiometric P removal range, a precipitation model describes the interactions between metal and phosphorus. However, at very low P concentrations, more complex models that include precipitation, adsorption and floc specific surface are required.
The benefits of sequential chemical addition for coagulation operations have been shown on many occasions. This is also the case with phosphorus precipitation. For very low final concentrations, overall coagulant dosages can be significantly reduced.
The degree of phosphorus removal depends not only on the coagulant added, but also on the mode of solid-liquid separation employed. This is particularly important for those cases where very low final phosphorus concentrations are achieved. Effluent suspended solids contribute significantly to effluent total phosphorus concentrations. For very low phosphorus residuals, and high metal coagulant dosages, the phosphorus content of effluent suspended solids is significantly reduced. The reason is that at very high metal dosages, a larger proportion of the precipitates formed are metal hydroxides.
Wastewater Treatment
Physical-chemical treatment of wastewater was widely practiced until the late 19th century, until the advent of the trickling filter for biological treatment. The early 1970s saw a partial revival of interest that has continued to the present day, particularly for treatment plants that are overloaded during peak flow events. The addition of coagulant chemicals to primary clarifiers, or to other dedicated physical separation processes, is an effective way of reducing the load to downstream biological processes, or in some cases for direct discharge. This practice is generally referred to as chemically enhanced primary treatment, or CEPT.
Principal disadvantages that might preclude a wholly physical-chemical solution to wastewater treatment are the problems associated with the highly putrescible sludgeproduced, and the high operating costs of chemical addition. However, much of the current interests in physical-chemical treatment stem from its suitability for treatment under emergency measures; for seasonal applications, to avoid excess wastewater discharges during storm events; and for primary treatment before biological treatment, where the above disadvantages become of lesser impact.
CEPT can also be an effective first step for pollution control in developing countries – particularly in large urban areas that have evolved with sewerage systems but without centralized wastewater treatment, and have limited financial resources for more complete, but capital intensive biological treatment options such as activatedsludge systems. Such urban developments also may not have the areas available for appropriate technology options such as stabilization pond processes.
The efficiency of CEPT, in terms of BOD or COD removal, depends on wastewater characteristics. With CEPT, one can expect to remove particulate components, together with some portion of the colloidal components. Therefore, with such a wastewater, it is feasible to achieve removals of more than: 95-percent TSS; 65-percent COD; 50-percent BOD; 20-percent nitrogen; and 95-percent phosphorus. In practice, removals may be lower or higher: for example, in warmer climates, with larger collection systems, and relatively flat sewers, one would expect a higher degree of hydrolysis of particulate matter resulting in higher soluble fractions, and lower overall removals with CEPT. On the other hand, if the collection system is relatively small, the climate is cold, and wastewater is relatively fresh, there may be a higher proportion of particulate material, and CEPT removals could be higher.
Staged coagulation-flocculation can enhance CEPT performance. For example, at primary clarifier overflow rates of over 6 m/h (3,600 gpd/ft2) during peak flow treatment, TSS and BOD removals of 80 to 95%, and 58 to 68% were achieved, respectively, using 60 mg/l ferric chloride, followed by 15 mg/l polyaluminum chloride, followed by 0.5 mg/l anionic polymer. The total reaction time from the point of ferric chloride addition to entering the primary clarifiers was approximately 8 minutes at peak flow.
Factors affecting coagulation operations
Temperature
Temperature significantly affects coagulation operations, particularly for low turbidity waters, by shifting the optimum pH. This can be mitigated by operating at an optimum pOH as given by:
pH + pOH = pKW; where pKW = 0.01706xT + 4470.99/T – 6.0875
and T = temperature in °K = 273.15 + °C.
One advantage of the pre-polymerized coagulants such as PACl and polyferric sulfate is that they potentially can be tailored for particular raw water conditions such as temperature and other parameters, and can be less sensitive to changes in temperature.
Sequence of chemical addition
Traditionally, the sequence of chemical addition for coagulation operations is to first add chemicals for pH correction, then add the metal coagulant, then add the flocculant aid. Not all these chemicals are necessarily added, but the sequence logic is often as described. However, there are instances when other sequences are more effective, including inverting the sequence of metal coagulant and polymer addition, and the sequence of metal coagulant addition and pH adjustment. The best sequence for a particular application can be determined by jar test experiments.
Residual aluminum
Residual aluminum in treated water is undesirable for aesthetic reasons, but also because of a possible link between aluminum and adverse neurological effects such as Alzheimer’s disease. Although ingestion from drinking water constitutes a relatively small proportion of daily intake, residual aluminum in treated waters can be minimized by proper adjustment of pH. Figure 5 shows the relationship between residual aluminum and treatment pH. However, the optimum pH to minimize residual aluminum also depends on other substances in solution. For example, the presence of fluoride in the raw water shifts the pH of minimum Al residual upwards towards 7, depending on the fluoride concentration.
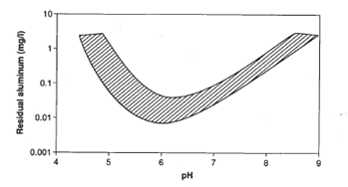
Figure 5. Compilation of residual aluminum determined from jar tests using membrane filters ranging from 0.05 to 0.45 µm (adapted from reference 3)
The presence of NOM also complicates the issue. Because of complexation of aluminum species with humic substances, the residual aluminum is linked to the removal of NOM. For example, at low alum dosages applied to humic waters, residual aluminum concentrations after treatment can be relatively high. At higher applied alum dosages, where a larger proportion of the humic substances are removed, residual Al concentrations after treatment are often significantly lower. This reduction in residual aluminum with higher aluminum dosages has also been found during fluoride removal.
The contribution of colloidal material to the aluminum residual emphasizes the importance of achieving low final treated water turbidities, at least less than 0.1 NTU, to minimize final aluminum residuals. When addressing high aluminum residuals, it is also important to determine whether the aluminum is in the particulate form, which would indicate improvements to filter retention, or whether it is soluble, which would require improving the chemistry of coagulation – particularly the pH beforefiltration.
Rapid Mixing
The rapid mixing stage is possibly the most important component of coagulation-flocculation processes, since it is here that destabilization reactions occur and where primary floc particles are formed, whose characteristics markedly influence subsequent flocculation kinetics. In general it is likely that the metal coagulant hydrolysis products that are formed within the time range 0.01 to 1.0 seconds are the most important for effective destabilization. In many instances, traditional 30 to 60 second retention times during rapid mixing are unnecessary and flocculation efficiency may not improve beyond rapid mix times of approximately 5 seconds or less. Indeed, beyond a certain optimum rapid mix time, a detrimental effect on flocculation efficiency may result.
The type of rapid mixer often installed in practice is given the general name back-mix reactors. These are often designed to provide a 10 to 60 second retention time with a root mean square velocity gradient, G, of the order 300 s-1. Back-mix reactors normally comprise square tanks with vertical impellers. In many instances these back-mix reactors have been abandoned or not used extensively due to the poor results often attained.
Other modes of rapid mixing include in-line mixers either with or without controlled velocity gradients. In general, in-line mixers provide the best rapid mixing conditions. In-line mixers without velocity gradient control include static mixers, orifice plates, diffuser grids in an open channel, and hydraulic jumps in open channels. In each of these cases, the velocity gradient and degree of mixing is dependent on the flow rate.
In-line mixers with velocity gradient control include in-line mechanical mixers with variable speed impellers, and in-line jet mixers. In the latter case, the velocity gradient is varied by the flow rate though the jet nozzle. Typically these are mounted concentrically within an enclosed pipe, with the jet discharging against the flow, as shown in Figure 6.

Figure 6. An example installation of an in-line jet rapid mixing device.
Flocculation
Orthokinetic flocculation arises from induced velocity gradients in the liquid. It is here that primary particles are induced to approach close enough together, make contact and progressively form larger agglomerates, or flocs. The principal parameter governing the rate of orthokinetic flocculation is the velocity gradient applied. The degree or extent of flocculation is governed by both applied velocity gradients and time of flocculation. These two parameters influence the rate and extent of particle aggregation and the rate and extent of breakup of these aggregates.
There are various ways to induce velocity gradients: baffled chambers; granular media beds; diffused air; spiral flow chambers; reciprocating blades and rotating blades.
For larger water treatment applications, rotating blades are the most common. Examples of rotating blade type flocculation devices are shown in Figure 7. Generally, such devices are of two types: horizontal shaft and vertical shaft.

Figure 7. Examples of rotating blade flocculators. Left: vertical paddle (courtesy Amwell); Center: horizontal (reel) paddle (courtesy WesTech); Right: axial flow (courtesy top-right: Anco; bottom-right: Philadelphia Mixers)
An advantage of horizontal shaft units is that compartmentalization is simple. With adjacent sections along the drive shaft carrying different configurations of agitators, different tapered G values are produced for one particular drive shaft rotational speed.
With most designs of horizontal shaft flocculators, the provision of stators within the flocculation basin to minimize rotation of the water with the blades is difficult.
The principal advantages of vertical shaft rotary devices are that underwater bearings are not required; the drive unit is above the water level and the arrangement for stators is simple. These are of two basic types, axial flow, and turbine flocculators. A disadvantage of turbine flocculators is that there is a much wider diversity of G values within the flocculation basin for a given mean velocity gradient – being high in the vicinity of the device and low near the walls of the basin. Such devices generally provide lower flocculation performance when compared with devices accommodating as much of the flocculation basin volume as possible.
Figure 8 compares paddle type, axial flow and turbine flocculators. There was extreme turbulence trailing the blades of the turbine device, whereas there was little turbulence at the blades of the axial flow device. The paddle type flocculation device relies on highly localized eddies trailing behind the blades, turbulent vortices in the corners of square basins, and the drag on the basin walls, for most of the power input for flocculation. The axial flow flocculating device appeared to be the preferred form of mechanical flocculation device.
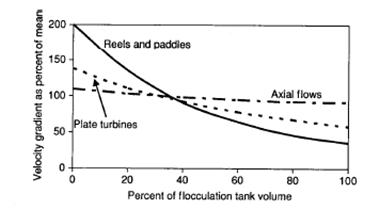
Figure 8. Spatial distribution of velocity gradients throughout the tank volume, for different flocculation devices4
Compartmentalization of flocculation tanks is beneficial. Short-circuiting is minimized, and a tapered velocity gradient profile can be imposed. By providing a high velocity gradient in the first chamber and successively lower velocity gradients in subsequent compartments, flocs with denser, more robust characteristics will be produced compared to a system where a single low G value is assigned to one or a series of tanks. A further beneficial effect with tapered velocity gradients is that the total retention time for a series of reactors is lower than a series of reactors subjected to the same mean velocity gradient in each reactor.
The transfer of flocculating water from one compartment to the next, or of flocculated water from the final compartment to a sedimentation or flotation basin, must be carried out so as to prevent excessive breakup of flocs already formed.
Testing and Control
The efficiency of the coagulation-flocculation process is dependent on many variables. For a particular water these may include:
- Type of coagulant used
- Coagulant dosage
- Final pH
- Coagulant feed concentration
- Type and dosage of chemical additives other than primary coagulant (e.g. polymers)
- Sequence of chemical addition and time lag between dosing points
- Intensity and duration of mixing at rapid mix stage
- Type of rapid mix device
- Velocity gradients applied during flocculation stage
- Flocculator retention time
- Type of stirring device used
- Flocculator geometry.
The basic apparatus used for assessing coagulation performance is the jar test apparatus, an example of which is shown in Figure 9.

Figure 9. Example of jar test apparatus. This particular model includes simultaneous chemical dosing to all six jars, with dual syringes; high intensity mixing with removable baffles; digital clock with count up/down timer alarms; 1-liter jars with sampling stopcocks; motor speed control with digital tachometer; illumination and cooling fan. (Courtesy EC Engineering)
The evaluation of optimum coagulant type, dosage and pH using the jar test procedure may be carried out on the basis of a wide variety of criteria; the applicability of each dependent on the particular circumstances and the processes used after coagulation. Common criteria include supernatant turbidity after settling, and filtrationthrough filter papers. However, there is a long list of possible criteria, each appropriate for the particular water under test, and for the existing or proposed treatment processes after coagulation and flocculation.
Figure 10 shows results obtained using the membrane refiltration technique, and Figure 11 shows the jar test paper filtration technique for assessing filtered water quality after coagulation and flocculation. Figure 12 shows results obtained using settled water turbidity as the criterion. Figure 13 shows results optimizing flocculator retention time.
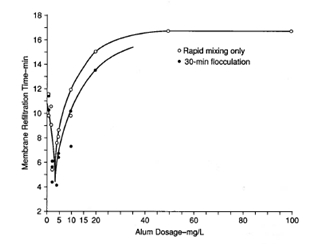
Figure 10. Example of membrane refiltration experiments. Each alum dosage was at the optimum pH; filtration was carried out with 47-mm membrane filters with 100-mm Hg vacuum; times recorded were those required to refilter 80 ml.1
Figure 11. Jar test filtration assembly. Filter paper folded twice to fit into funnel. (Suitable papers are 125 mm Whatman No. 40).1
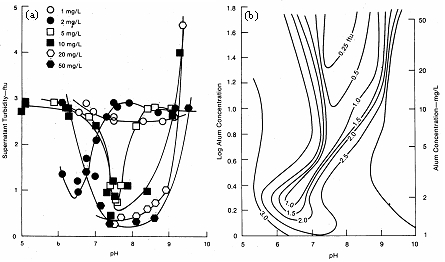
Figure 12. Results from jar test (settling) experiments.1
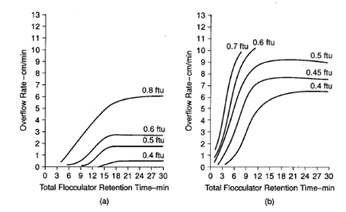
Figure 13. Settling characteristics for a range of total flocculator retention times T (T1 = T2 = T3 = T/3). (a) Alum used alone, dosage 15 mg/l at pH 7.6; rapid mix 500s-1 for 10 s; N0(average) 1.9 NTU; raw turbidity (average) 1.5 NTU; G1, G2 and G3 80, 25 and 12.5 s-1 resp. (b) Alum with polymer. Alum dosage 3 mg/l at pH 6.4; rapid mix for alum 500 s-1 for 10 s; preflocculation 100 s-1 for 10 min; poly dosage 0.15 mg/l; rapid mix for poly 100 s-1 for 10 s; N0(average) 0.88 NTU; raw water turbidity (average) 2.32 NTU; G1, G2 and G3 100, 50 and 30 s-1 respectively.1
The basic jar testing technique can be used effectively to determine optimum conditions for flocculation, including G values and flocculation times. It can also be used to evaluate settling characteristics after coagulation and flocculation pretreatment.
Recent developments include automated jar testing apparatus for testing a large number of pre-programmed coagulation chemicals and pH conditions.
Control systems for coagulation operations include streaming current and floc formation monitors. A new class of emerging control strategies include data-driven systems such as fuzzy logic and artificial neural network controllers.
Sludge Handling
Some two million dry tons per year of alum sludge, and 0.3 million dry tons per year of ferric sludge were produced in water treatment plants in the US in 1971. Rigorous legislation is now in effect in several countries to limit the impacts of such residuals on the environment. The internal recycling of in-plant residuals is now regulated in many places. Water sludges are generally characterized by BOD concentrations in the hundreds and COD concentrations in the thousands of mg/l.
Water sludges generally require some form of conditioning ahead of mechanical thickening and dewatering. Exceptions are natural freezing operations and sludge storage in lagoons. Sand drying beds also can condition sludge with polymer to accelerate drainage. Solar drying beds are typically operated without conditioning. Thickening and dewatering are generally required prior to coagulant recovery.
There are a number of disposal routes for water plant sludges. These include lagooning, land application, landfill, disposal to sewers and co-disposal with wastewaterplant sludges.
Further Reading
David W. (2010) Hendricks Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological, IWA Publishing.
Benchmarking of Control Strategies for Wastewater Treatment Plants – Krist V Gernaey, Ulf Jeppsson, Peter A Vanrolleghem, John B Copp and Jean-Philippe Steyer
Publication Date: Feb 2013 – ISBN – 9781843391463
Sustainable Food Waste Evaluation – David L. Parry
Publication Date: Feb 2013 – ISBN – 9781780401287
Handbook of Environmental Odour Management – Franz-Bernd Frechen, Richard M. Stuetz, Anton P. van Harreveld, Jean-Michel Guillot
Publication Date: Jan 2013 – ISBN – 9781780400600
Gathering Unpublished Data for Chemicals Detected in Biosolids – Charles Pittinger, Andrew Maier and Drew C. McAvoy,
Publication Date: Jan 2013 – ISBN – 9781780401102
Barriers to Biogas Use for Renewable Energy – WERF
Publication Date: Dec 2012 – ISBN – 9781780401010
Attenuation of PPCP/EDCs Through Golf Courses Using Recycled Water – Michael D. McCullough
Publication Date: Nov 2012 – ISBN – 9781780403977
References
- Bratby J. (2006) Coagulation and Flocculation in Water and wastewater Treatment. IWA Publishing, London, Seattle.
- Akers R.J. (1972) Flocculation. Institute of Chemical Engineers, London.
- Jekel M.R., Heinzmann B. (1989) Residual aluminum in drinking-water treatment. JWSRT-Aqua, 38, 281-288.
- Hudson H.E. (1981) Water clarification processes. Practical design and evaluation. Van Nostrand Reinhold, New York.
Related Publications
Evolution of Sanitation and Wastewater Technologies through the Centuries – Andreas N. Angelakis, Peter A. Wilderer and Joan Bray Rose
Publication Date: Mar 2014 – ISBN – 9781780404844
Experimental Methods in Wastewater Treatment – M.C.M. van Loosdrecht, J. Keller, P.H. Nielsen, C.M. Lopez-Vazquez and D. Brdjanovic
Publication Date: Feb 2014 – ISBN – 9781780404745
Faecal Sludge Management – Linda Strande, Mariska Ronteltap and Damir Brdjanovic
Publication Date: Nov 2013 – ISBN – 9781780404721
Benchmarking of Control Strategies for Wastewater Treatment Plants – Krist V Gernaey, Ulf Jeppsson, Peter A Vanrolleghem, John B Copp and Jean-Philippe Steyer
Publication Date: Sep 2013 – ISBN – 9781843391463
